PCAF: The Rule
go.ncsu.edu/readext?513011
en Español / em Português
El inglés es el idioma de control de esta página. En la medida en que haya algún conflicto entre la traducción al inglés y la traducción, el inglés prevalece.
Al hacer clic en el enlace de traducción se activa un servicio de traducción gratuito para convertir la página al español. Al igual que con cualquier traducción por Internet, la conversión no es sensible al contexto y puede que no traduzca el texto en su significado original. NC State Extension no garantiza la exactitud del texto traducido. Por favor, tenga en cuenta que algunas aplicaciones y/o servicios pueden no funcionar como se espera cuando se traducen.
Português
Inglês é o idioma de controle desta página. Na medida que haja algum conflito entre o texto original em Inglês e a tradução, o Inglês prevalece.
Ao clicar no link de tradução, um serviço gratuito de tradução será ativado para converter a página para o Português. Como em qualquer tradução pela internet, a conversão não é sensivel ao contexto e pode não ocorrer a tradução para o significado orginal. O serviço de Extensão da Carolina do Norte (NC State Extension) não garante a exatidão do texto traduzido. Por favor, observe que algumas funções ou serviços podem não funcionar como esperado após a tradução.
English
English is the controlling language of this page. To the extent there is any conflict between the English text and the translation, English controls.
Clicking on the translation link activates a free translation service to convert the page to Spanish. As with any Internet translation, the conversion is not context-sensitive and may not translate the text to its original meaning. NC State Extension does not guarantee the accuracy of the translated text. Please note that some applications and/or services may not function as expected when translated.
Collapse ▲The PCAF rule can be overwhelming for many. This page provides a general overview of the PCAF rule and the subparts of the rule with resources to help you better understand the components of the rule and its subparts. This is important for understanding how they fit together to improve food safety standards.
There are six parts of the PCAF rule:
Subpart A: General Provisions
Subpart B: Current Good Manufacturing Practice
Subpart C: Hazard Analysis and Risk-Based Preventive Controls
Subpart D: Withdrawal of a Qualified Facility
Subpart E: Supply Chain Program
Subpart F: Requirements Applying to Records That Must Be Established and Maintained
For an overview of how PCAF fits in with FDA regulations related to animal food, see our diagram below.
Subpart A: General Provisions
Subpart A covers who must comply with PCAF and gives some definitions of the language used throughout the rule.
Who must comply?
Facilities that manufacture, process, pack, or hold animal food for consumption in the United States. In general, those that register under the Bioterrorism Act of 2002. Animal food covered by specific CGMP regulations must still comply with those regulations (Low-acid canned food, Medicated feed).
Feed mills that are part of a farm are exempt from registering as a food facility and are not subject to the rule. For a feed mill to be part of a farm, the animals and feed mill are under the same management in one general location, and the animal food made at the mill is only fed to animals under the farm’s management. See farm definitions.
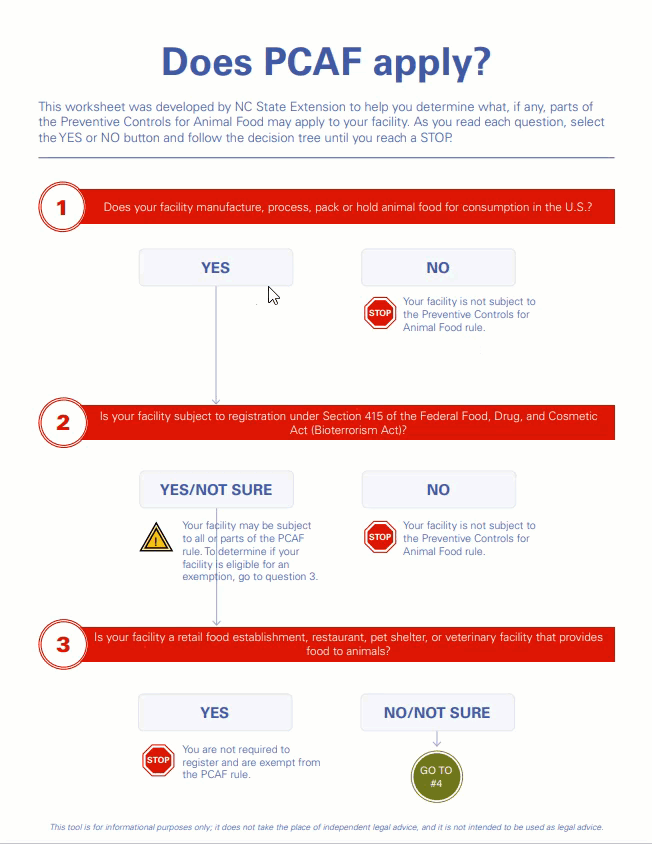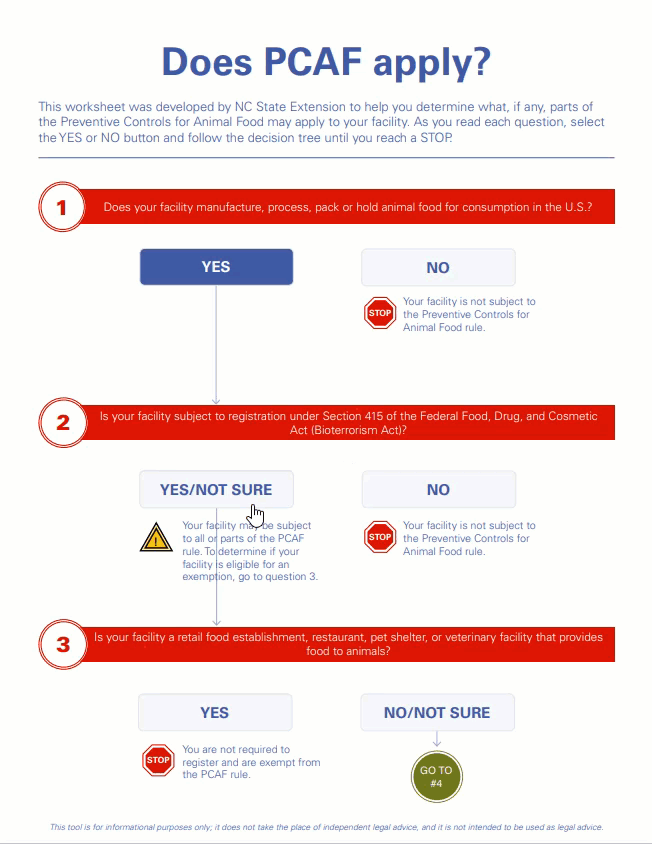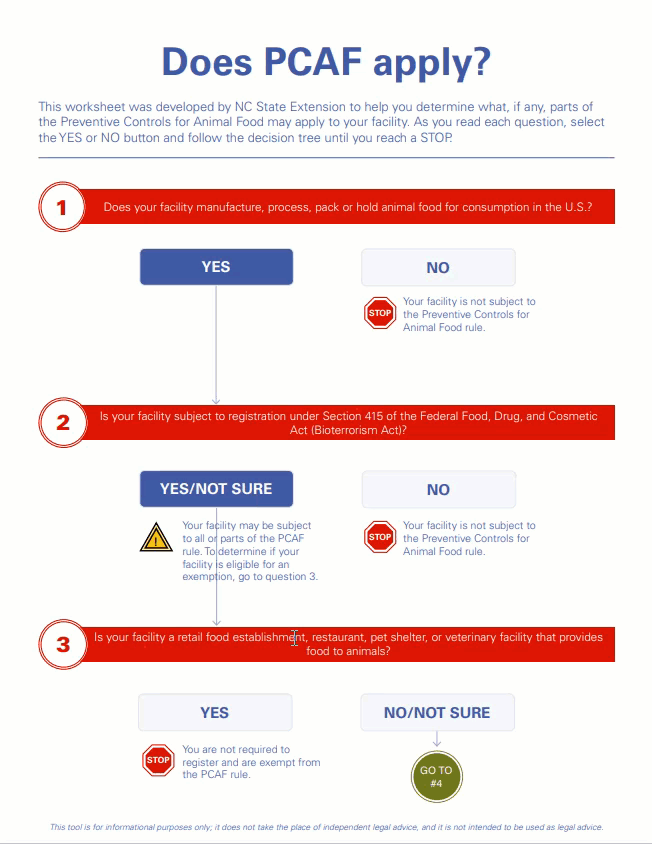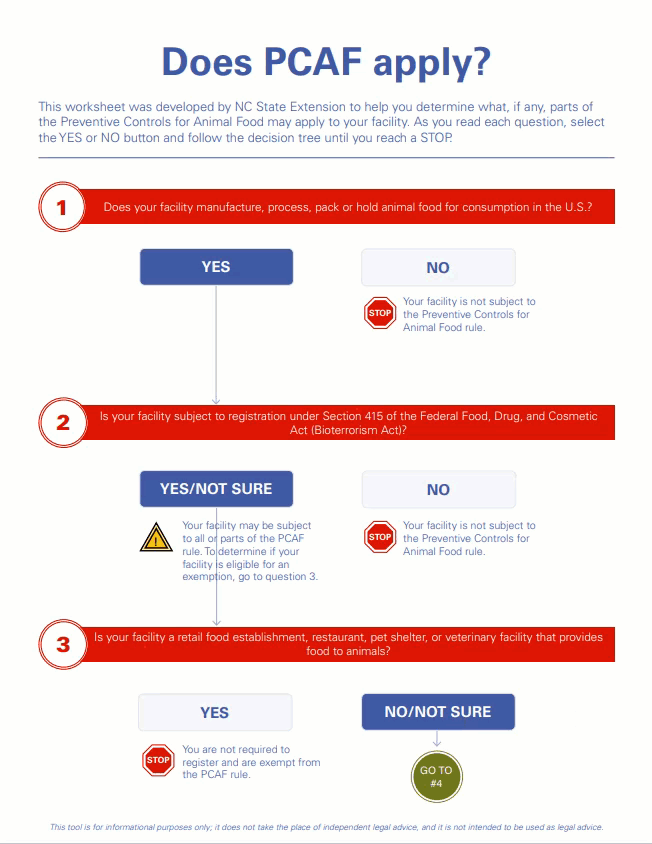
Our new interactive decision tree, “Does PCAF Apply?” is a short, easy-to-use tool for answering this important question.
The tool is for informational purposes only; it does not take the place of independent legal advice, and it is not intended to be used as legal advice.
FSMA Preventive Controls for Animal Food (PCAF) Exemption Decision Tree Tool
The NC State Feed Milling and Animal Food Safety programs have developed a decision tree tool to help guide facilities through the exemption determination process and the requirements for obtaining a qualified facility exemption through the U.S. Food and Drug Administration (FDA).
Subpart B: Current Good Manufacturing Practice
The Current Good Manufacturing Practice (CGMP) sets baseline standards for producing safe animal food and creating a successful food safety plan.
Learn more on our Current Good Manufacturing Practice page.
Subpart C: Hazard Analysis and Risk-Based Preventive Controls
Subpart C is the food safety plan requirement of the rule. There are multiple parts of subpart C and the food safety plan that are required.
Depending on the results of the hazard analysis – a required component for facilities that have to comply with subpart C – facilities may have additional requirements on top of the general food safety plan pieces.
Learn more on our Food Safety Plan, Hazard Analysis and Preventive Controls pages.
Food safety plans
A written FSP is required, unless the facility qualifies for an exemption, and must include the hazard analysis and applicable implementation records.
The food safety plan (FSP) must be written, or signed off on, by a Preventive Controls Qualified Individual (PCQI), who is qualified through training or job experience.
If the hazard analysis determines there is a hazard requiring a preventive control, the food safety plan must also include preventive controls and their management components, and a recall plan.
The food safety plan and hazard analysis formats are flexible to meet the needs of the facility. The food safety plan must be reanalyzed at least every three (3) years or more frequently, as appropriate.
Recall plans are a requirement of the food safety plan if there are any hazards requiring preventive controls identified in the hazard analysis. The plan must be written and describe steps to take and assign responsibility to notify directly customers and consignees, notify the public, when appropriate, conduct effectiveness checks, and execute disposition of the food.
Get help with your food safety plan.
Subpart E: Supply-Chain Program
The Supply-Chain Program, a special type of preventive control, is used when a receiving facility decides to have their supplier control hazards in an incoming ingredient or food.
This program is appropriate when hazards are controlled prior to receipt by the receiving facility that manufactures/processes the animal food. It establishes specific requirements that the receiving facility must have in place in order to assure that a supplier program is sufficient to protect animal food safety.
The Supply-Chain Program subpart can be very complex and requires extensive documentation and verification of monitoring, corrective actions, and implementation and effectiveness.
Subpart F: Records
Records must be kept as original records, true copies, or electronic records, can contain actual values and observations, must be accurate, indelible, and legible, must be created concurrently with performance of the activity, and must be as detailed as possible. Records must include information adequate to identify the
- plant or facility (name and location),
- date, and when appropriate, time of the activity,
- signature or initials of the person performing the activity, and
- the identity of the product and lot code, if any, when appropriate.
These records are exempt from 21 CFR 11 requirements regarding electronic records and electronic signatures.
The FDA has provided a guidance document for What You Need To Know About Establishment, Maintenance, and Availability of Records.
For additional information contact
Marissa Herchler Cohen
NC State University
Cooperative Extension Service
Prestage Department of Poultry Science
234 D Scott Hall
Raleigh, NC 27695-7608
E-mail: marissa_cohen@ncsu.edu
Phone: +1 919.515.5396