PCAF: Current Good Manufacturing Practice
go.ncsu.edu/readext?755975
en Español / em Português
El inglés es el idioma de control de esta página. En la medida en que haya algún conflicto entre la traducción al inglés y la traducción, el inglés prevalece.
Al hacer clic en el enlace de traducción se activa un servicio de traducción gratuito para convertir la página al español. Al igual que con cualquier traducción por Internet, la conversión no es sensible al contexto y puede que no traduzca el texto en su significado original. NC State Extension no garantiza la exactitud del texto traducido. Por favor, tenga en cuenta que algunas aplicaciones y/o servicios pueden no funcionar como se espera cuando se traducen.
Português
Inglês é o idioma de controle desta página. Na medida que haja algum conflito entre o texto original em Inglês e a tradução, o Inglês prevalece.
Ao clicar no link de tradução, um serviço gratuito de tradução será ativado para converter a página para o Português. Como em qualquer tradução pela internet, a conversão não é sensivel ao contexto e pode não ocorrer a tradução para o significado orginal. O serviço de Extensão da Carolina do Norte (NC State Extension) não garante a exatidão do texto traduzido. Por favor, observe que algumas funções ou serviços podem não funcionar como esperado após a tradução.
English
English is the controlling language of this page. To the extent there is any conflict between the English text and the translation, English controls.
Clicking on the translation link activates a free translation service to convert the page to Spanish. As with any Internet translation, the conversion is not context-sensitive and may not translate the text to its original meaning. NC State Extension does not guarantee the accuracy of the translated text. Please note that some applications and/or services may not function as expected when translated.
Collapse ▲The Current Good Manufacturing Practice (CGMP) subpart of the PCAF rule is part of the regulations introduced by the FDA after passage of the Food Safety Modernization Act (FSMA) in 2011.
For an overview of how the CGMP subpart fits in with the PCAF rule, see our diagrams below.
Food Safety Modernization Act Current Good Manufacturing Practices for Food for Animals and COVID-19
How Animal Food Facilities Can Prepare for Regulatory Inspections
Why is CGMP Necessary?
According to the FDA, about 48 million people (1 in 6 Americans) get sick, 128,000 are hospitalized and 3,000 die each year from foodborne diseases. The administration argues that “this is a significant public health burden that is largely preventable.”
The FDA considers Current Good Manufacturing Practice (CGMP) to be
“necessary to prevent animal food from containing filthy, putrid, or decomposed substances, being otherwise unfit for food, or being prepared, packed, or held under insanitary conditions whereby it may have become contaminated with filth, or whereby it may have been rendered injurious to health.” (Preamble, II: Legal Authority).
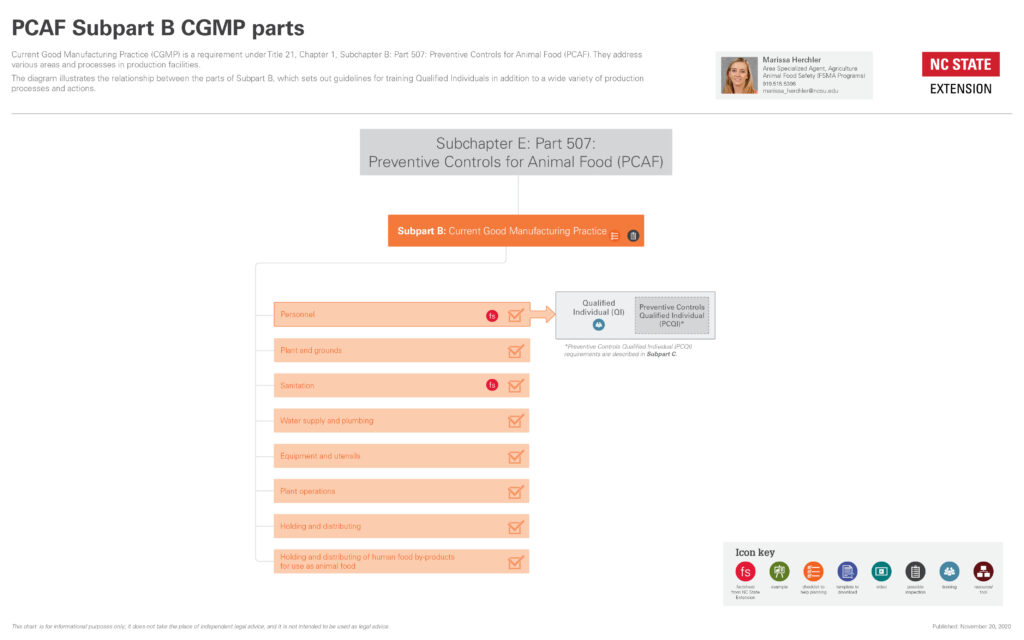
What are the Parts of CGMP?
The components of CGMP, listed in the rule are
- personnel,
- plant and grounds,
- sanitation,
- water supply and plumbing,
- equipment and utensils,
- plant operations,
- holding and distribution, and
- holding and distribution of human food by-products for use as animal food.
Our checklist can help you self-audit your facility.
Compliance Policy Guide
The FDA compliance policy guide linked below may be helpful when developing food safety plans. See other FDA compliance policy guides related to food safety plans.
For Additional Information Contact
Marissa Herchler Cohen
NC State University
Cooperative Extension Service
Prestage Department of Poultry Science
234 D Scott Hall
Raleigh, NC 27695-7608
E-mail: marissa_cohen@ncsu.edu
Phone: +1 919.515.5396