PCAF: Hazard Analysis
go.ncsu.edu/readext?755984
en Español / em Português
El inglés es el idioma de control de esta página. En la medida en que haya algún conflicto entre la traducción al inglés y la traducción, el inglés prevalece.
Al hacer clic en el enlace de traducción se activa un servicio de traducción gratuito para convertir la página al español. Al igual que con cualquier traducción por Internet, la conversión no es sensible al contexto y puede que no traduzca el texto en su significado original. NC State Extension no garantiza la exactitud del texto traducido. Por favor, tenga en cuenta que algunas aplicaciones y/o servicios pueden no funcionar como se espera cuando se traducen.
Português
Inglês é o idioma de controle desta página. Na medida que haja algum conflito entre o texto original em Inglês e a tradução, o Inglês prevalece.
Ao clicar no link de tradução, um serviço gratuito de tradução será ativado para converter a página para o Português. Como em qualquer tradução pela internet, a conversão não é sensivel ao contexto e pode não ocorrer a tradução para o significado orginal. O serviço de Extensão da Carolina do Norte (NC State Extension) não garante a exatidão do texto traduzido. Por favor, observe que algumas funções ou serviços podem não funcionar como esperado após a tradução.
English
English is the controlling language of this page. To the extent there is any conflict between the English text and the translation, English controls.
Clicking on the translation link activates a free translation service to convert the page to Spanish. As with any Internet translation, the conversion is not context-sensitive and may not translate the text to its original meaning. NC State Extension does not guarantee the accuracy of the translated text. Please note that some applications and/or services may not function as expected when translated.
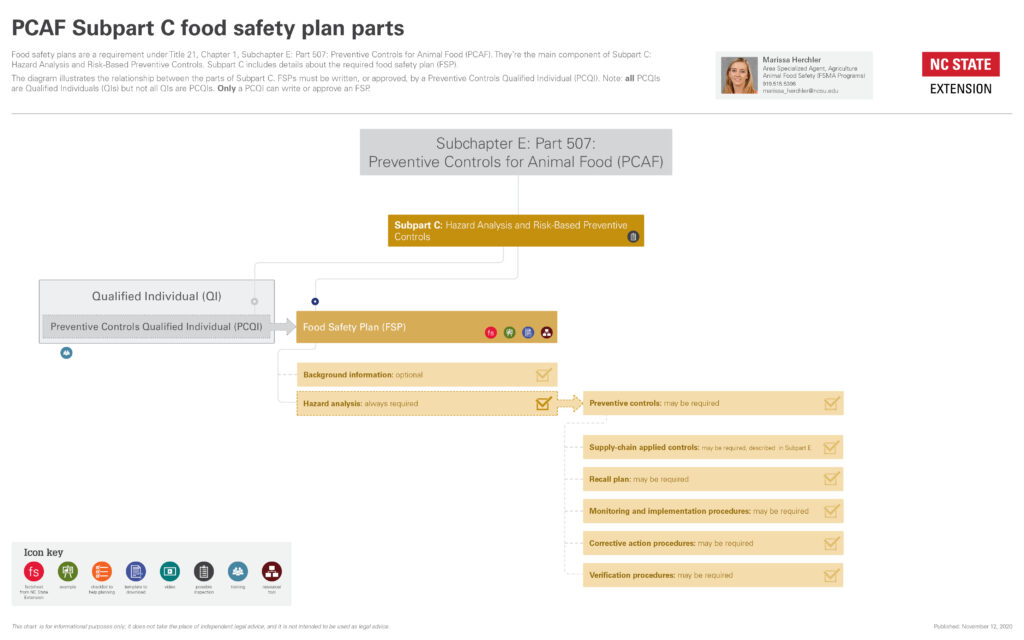
Collapse ▲Hazard analysis is part of the regulations introduced by the FDA after passage of the Food Safety Modernization Act (FSMA) in 2011. It’s described in Subpart C of the Preventive Controls for Animal Food (PCAF) rule.
For an overview of how hazard analysis fits in with the PCAF rule, see our diagrams below.
If you are required to implement a food safety plan, a hazard analysis is always required to be part of the food safety plan.
The table below lists the management components you may need to include in your food safety plan, depending on the results of your hazard analysis.
| Management components for ensuring effectiveness of different controls | |
| Recall plan |
If you identify a PC in your FSP, you must include a recall plan in your food safety plan. A recall plan is always a good business practice, whether it is needed in the food safety plan or not. |
| Monitoring and implementation procedures | Required for process PCs and sanitation PCs. |
| Corrective action procedures and corrections | If you identify a PC in your FSP, you must include corrective action procedures in your food safety plan. |
| Verification procedures | Required if a PC is identified in the hazard analysis. |
| Validation |
Required for process PCs. Validation is not required for sanitation PCs because it is expected the manufacturers of sanitation solutions will validate the effectiveness of the product. |
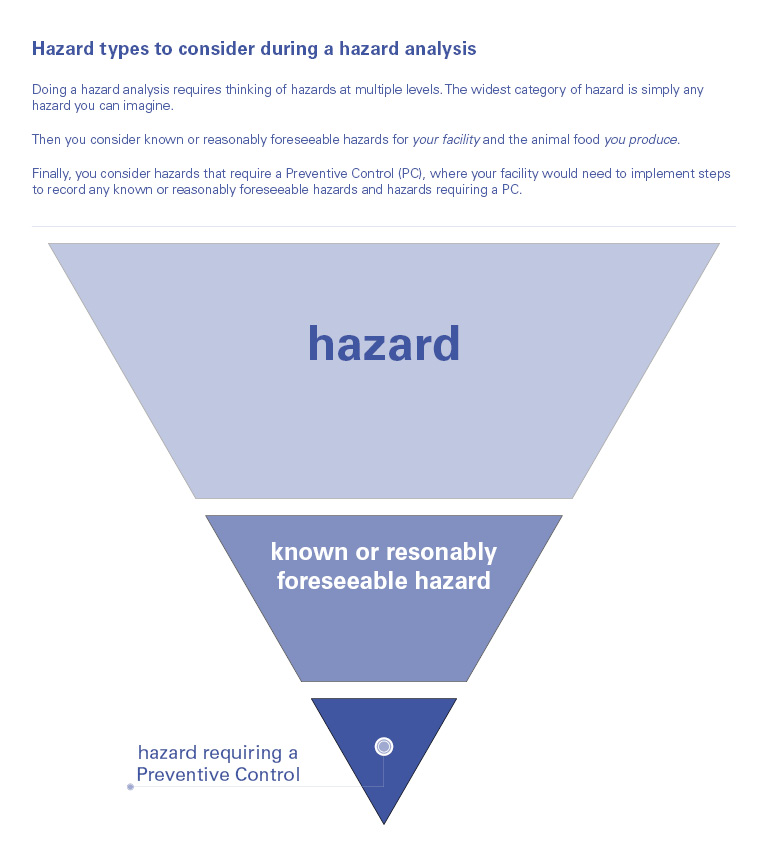
It’s important to note that the hazard analysis is not one-size-fits-all. There are going to be differences in known or reasonably foreseeable hazards based on the species, production class and how the animals are fed. There will also be differences in preventive controls determinations, as different PCQIs will determine different severity, probability and prerequisite programs and CGMP activities per facility and based on their experiences.
There are some items that must be considered in the hazard analysis:
- formulation of the animal food,
- condition, function, and design of facility and equipment,
- raw materials and other ingredients,
- transportation practices,
- manufacturing/processing procedures,
- packaging and labeling activities,
- storage and distribution,
- intended or reasonably foreseeable use,
- Sanitation, including employee hygiene, and
- other relevant factors, such as temporal (weather-related) nature of some hazards.
What is a Hazard?
A hazard, as defined by the FDA, is any biological, chemical (including radiological) or physical agent that has the potential to cause illness or injury in humans or animals.
Some example hazards in animal food include:
- Biological Hazards (Salmonella spp., Listeria monocytogenes),
- Chemical Hazards (mycotoxins, pesticides and process-related or industrial chemicals, drug carryover, nutrient deficiencies or toxicities), and
- Physical Hazards (stones, glass, metal).
Note: This is not an exhaustive list of hazards and should be considered examples. Each facility should go through an extensive hazard analysis to determine the appropriate hazards for their facility and food processed.
Aflatoxins and Animal Food Safety
For additional information contact
Marissa Herchler Cohen
NC State University
Cooperative Extension Service
Prestage Department of Poultry Science
234 D Scott Hall
Raleigh, NC 27695-7608
Email: marissa_cohen@ncsu.edu
Phone: +1 919.515.5396